Standardized Whole-Blood Immunophenotyping Panels on Flow Cytometry for Transplant Recipients and Clinical Trials
Elvira Jimenez Vera1, Yi Vee Chew1, Heather Burns1, Patricia Anderson1, Lindy Williams1, Suat Dervish2, Xin Maggie Wang2, Shounan Yi1, Wayne Hawthorne1, Stephen Alexander3, Philip O'Connell1, Min Hu1.
1Centre for Transplant and Renal Research, Westmead Hospital, Westmead Institute for Medical Research, Westmead, Australia; 2Flow Cytometry Core Facility, Westmead Institute for Medical Research, Westmead, Australia; 3Centre for Kidney Research, Children's Hospital at Westmead, University of Sydney, Westmead, Australia
Introduction: Immunophenotyping of whole blood by flow cytometry is a reliable, fast, and easy method used to obtain a large amount of information on the effects and outcomes of different treatments in transplantation on immune phenotype with minimal impact on the patient. Aim: 1) To establish whole-blood immunophenotyping panels for transplant recipients, 2) To develop a concise method involving the standardisation of reagents, sample handling, instrument setup and data analysis.
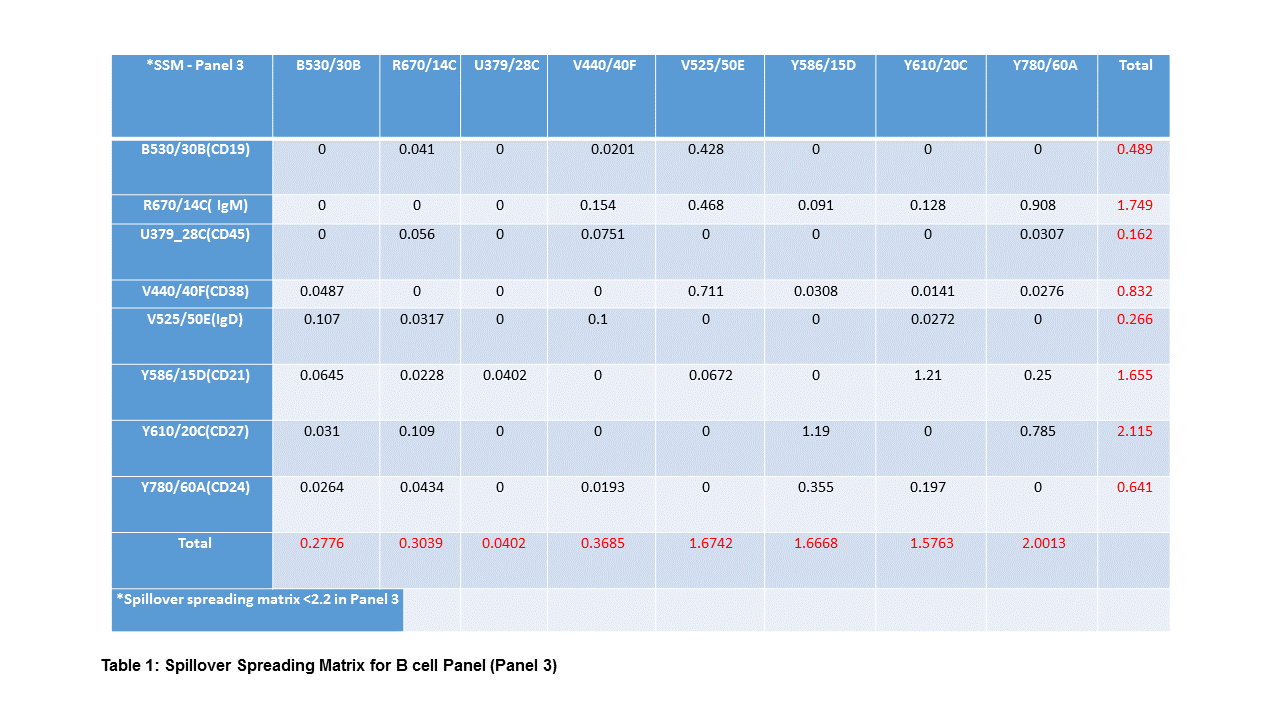
Methods: Absolute cell count (TruCount) and seven leukocyte-profiling panels containing 8-10 marker-antigens (46 of which were unique within the panels) consisting of subsets and/or status of granulocytes, monocytes, DCs, B , NK, , and T cells including Tregs and NKT were used to monitor the immune profiles of paediatric kidney transplant and adult islet cell transplant recipients. Whole-blood samples were stained and acquired on a BD-LSRFortessa and Flowjo was used for data analysis. BD™ Cytometer Setup and Tracking beads monitored cytometer performance. Sample staining was performed within 2 hours of blood sample collection. The accuracy and variability of these panels were determined and 100-300 µl whole-blood was used for each panel. Results: The 46 antibodies were titrated and the staining index (SI) was calculated (CD45BUV395 in Fig.1A) for panel optimisation. Application settings on a BD LSRFortessa, measurement of the spillover spreading matrix (SSM) for each panel [SSM in Panel 3 (Tab.1)], and optimal antibody quantity for the panel-cocktail were established. Auto-analysis templates and gating strategies (Panel 1 in Fig.1B) to target subsets of immune-cells were set up and blood sample collection, preparation, antibody cocktails, and staining protocols were standardised. 9 paediatric kidney transplant patients, 4 islet transplant patients (which are to be followed until two years post third islet transplant), 13 T1D patients and 8 control samples have been evaluated to date. The ability to identify consistent immune subsets across all panels over 6 months (Fig.1C from TruCount Panel) was achieved, and was used to longitudinally track the proportions of cell populations in transplant patients (Fig.1D Panel 1).
Conclusion: We standardised immune panels and procedures for absolute cell numbers and multiple subsets of immune cells for monitoring a range of individuals including healthy controls, paediatric kidney recipients, T1D, and islet transplant recipients. We have demonstrated that immunophenotyping by flow cytometry is a reliable, consistent, and fast technique that allows the detection of changes in absolute cell numbers of leukocyte subsets from any recipients’ whole blood. This immunophenotyping by flow cytometry is a non-invasive technique which requires less than 1.5ml of whole blood and may be a useful tool for monitoring changes in the immune profile of individuals in a range of clinical trials.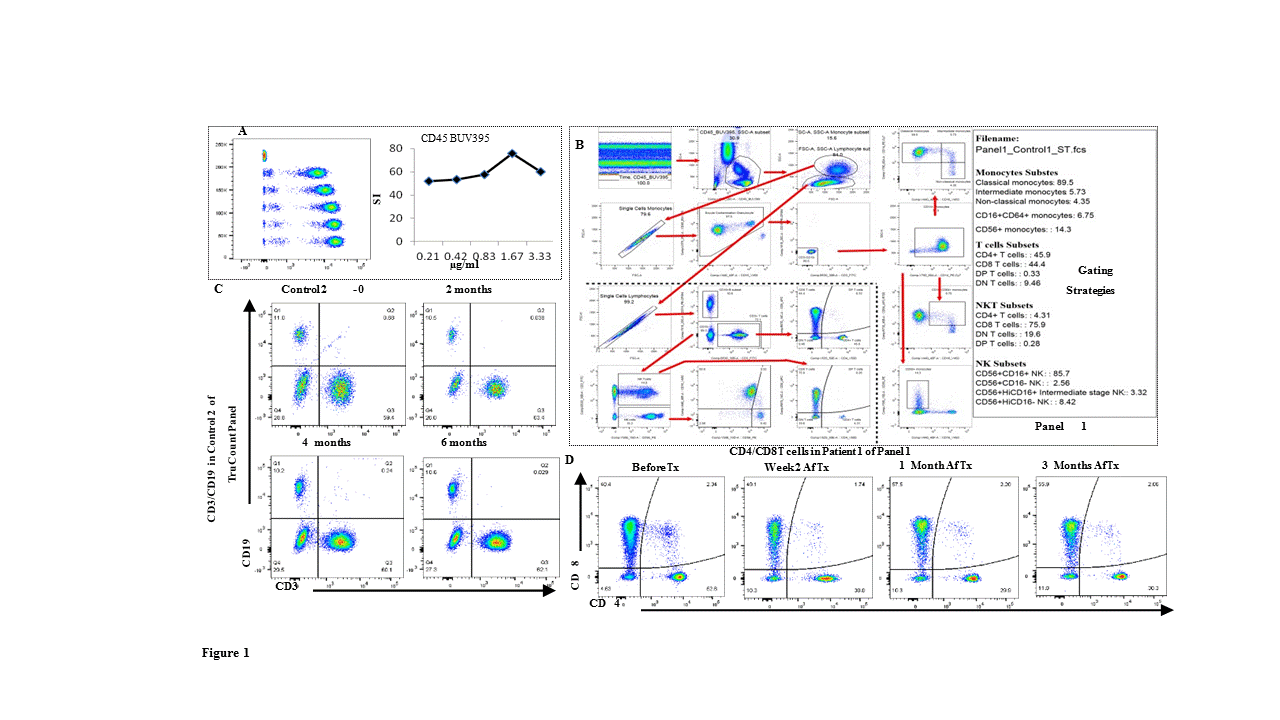
Birgit Sawitzki. Mathias Streitz.
